Can a Keto tautomer turn into its Enol isomer WITHOUT the presence of an acid or base? Like can the oxygen on the keto tautomer just grab its own alpha hydrogen? or are the oxygen and alpha hydrogen too far apart from each other?
Thank you guys! I feel like I'm getting conflicting information between my book and the interweb.
For example, can an amide be said to be in the keto form, and is the tautomerized version of the amide still called an enol even though it does not contain a C=C double bond (but rather a double bond to a nitrogen)?

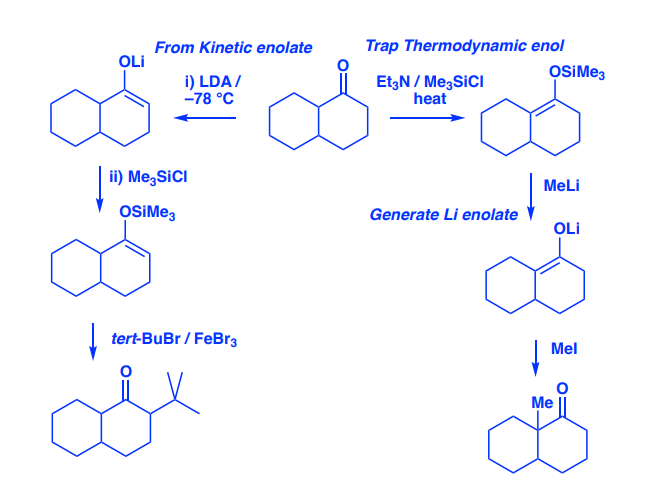
Okay, lets take another swing at this. I posted earlier this morning half awake, just to realize I really screwed up on my mechanism. So I just came back to this and lets try this again. Would this be a valid mechanism for the following problem?
https://preview.redd.it/7msiad15tpt51.jpg?width=1031&format=pjpg&auto=webp&s=edce88c3ebf77b5a3a9d6f1d3f0fe37c61e451b8
https://preview.redd.it/g5y64kn7tpt51.png?width=1284&format=png&auto=webp&s=910eef007b51f16be55d2357ce3d1ec1eab45ca5
Since enols are weak nucleophiles, is there a chance that this occurs which may interfere with the reaction?
Hey guys, I read online that in conditions of pH < pKa, keto form is preferred over enol form due to protonation. Can someone please explain what this means?? I thought keto form was referring to aldehydes and ketones and enol was referring to alcohol, so wouldn't an alcohol be protonated?

I just learned that monosaccharides are in their keto form when it’s linear and in their enol form when they are cyclic. Does this mean that sugars are more happy and stable in their linear form? This seems weird to me since rings seem more stable but I learned that keto forms are better..? Thank you.
Most sites tell me that it is because the alpha hydrogen is acidic but why is this so? In carboxylic acids, it is because the conjugate base is "resonance stabilised". Resonance does not stabilise the conjugate base per se. It is the delocalisation that confers the extra stability. However, to my knowledge, tautomerisation is not resonance. Therefore, there is no delocalisation in between keto and enol forms (otherwise they would be resonance forms of each other) Why then is the alpha hydrogen so acidic compared to an alkane?
If something is acidic it would mean its conjugate base is stabilised by something. But since that something is not resonance, why is the alpha hydrogen acidic? It is not to say that the C-H bond is particularly polar. (Note that I am not asking for the definition of a strong acid)
Thanks for reading!
As title. My teacher just simply said because 5-bromouracil is alike thymine in enol form so it switches, which doesn't explain the cause of the tautomerization.
I have a hard time logically understanding why the keto form is so much more prevalent than the enol. After a lot of reading, I came up with this: The C=O bond is stronger than the C=C bond, because the pi bond is very distorted with the electrons staying closer to the O. The carbon has enough electronegativity as to not want to let go of his electrons. Also, C and O are more attracted towards each other by being dipoles.(this one sounds weird even to me).
Am I right? Or could someone please give me a more logical explanation? Thanks guys/gals.
I have uploaded an image with my attempt at the keto and enol forms of a given molecule, which is posted here: http://i.imgur.com/vfhVfEn.png
Would I be right to assume that for the keto form you simply place the double bond at the oxygen and remove the H? And for the enol form you remove the double bond from the oxygen and add a hydrogen making it a OH group and place the double bond at either carbon next to the carbonyl carbon it as long as both carbons are alpha carbons with at least one enolisable hydrogen?
Also would it be right that for a given molecule such as shown in image 3, the carbanion form of the enolate ion can either be on either carbon as shown since there are two alpha carbons both with enolisable hydrogens? Thanks
I have 2 questions:
- I don't really get how the arrows from the hydrazone to the enehydrazine should be drawn
- Out of interest, which one would be the most stable structure?
https://preview.redd.it/ies58t8qtzw71.png?width=648&format=png&auto=webp&s=78e65ff967782f224a30148b59d5eba0dfe3d126
Journal of the American Chemical SocietyDOI: 10.1021/jacs.1c02833
Tetsuya Ezawa, Yoshihiro Sohtome, Daisuke Hashizume, Masaya Adachi, Mai Akakabe, Hiroyuki Koshino, and Mikiko Sodeoka
https://ift.tt/3wcEsnM
tautomerism: the condition, quality, or relation of metameric substances, or their respective derivatives, which are more or less interchangeable, according as one form or the other is the more stable. it is a special case of metamerism
See tree for tautomerism: https://treegledictionary.org/define/tautomerism
Is tautomerism a rearrangment reaction ?
On enut frren lote ge ot meqen

Thanks :)





A rare and most definitely obscure find.
https://preview.redd.it/3awn09vdiwk71.jpg?width=960&format=pjpg&auto=webp&s=36193b58f551303a532f065a06ec20a9bf2da964
https://preview.redd.it/1g8w28vdiwk71.jpg?width=1280&format=pjpg&auto=webp&s=27754dc18fe59e4444ca516be9a237030a831d49
tautomerism: the condition, quality, or relation of metameric substances, or their respective derivatives, which are more or less interchangeable, according as one form or the other is the more stable. it is a special case of metamerism
See tree for tautomerism: https://treegledictionary.org/define/tautomerism


